Background
We previously showed that low-dose immune tolerance induction (LD-ITI) strategy [incorporating immunosuppressants (IS) partially] for severe hemophilia A (SHA) patients with high-titer inhibitors (HTI) had relatively equivalent success rate with lower cost compared to those on high-dose ITI from international (I)-ITI randomized clinical trial (RCT). However, it is lack of RCT to compare the efficacy, safety, and cost of LD-ITI strategy to higher-dose ITI [intermediate-dose (MD)-ITI] strategy in China. Currently, the domestic recombinant FVIII (rFVIII) sponsored by the SinoCelltech Ltd., provides an opportunity to conduct the LOME study: a multicenter controlled randomized clinical trial of LOw- versus InterMEdiate-dose immune tolerance induction with recombinant factor VIII in China, to answer this question.
Objectives
Conduct the RCT to compare the efficacy, safety and cost of LD- to MD-ITI strategy using rFVIII (Omfiloctocog alfa, SCT800) for SHA children with HTI in China.
Methods
A perspective, multicenter RCT, enrolled SHA children ≤8year-old at enrollment, with peak historical inhibitor-titer 5 - 200 Bethesda Unit (BU)/mL, 1:1 randomized in LD- (Omfiloctocog alfa, rFVIII 50 IU/kg once-every-day) or MD- (Omfiloctocog alfa, rFVIII 100IU/kg/day) ITI group.
IS was added (the initial regimen) in patients who met the criteria as follows: (i) the inhibitor titer during ITI increased to ≥200 BU/mL; (ii) the peak titer did not appear within 3 months after ITI-start; (iii) inadequate reduction (<20%) in titer over 6 months.
Success: negative inhibitor titer twice consecutively at least 4 weeks apart and FVIII recovery ≥66% of expected within 24 months of treatment. Partial success: negative inhibitor titer twice consecutively at least 4 weeks apart, but with persistently abnormal FVIII recovery.
Average consumption cost (per kg body weight) was calculated based on the median number of treatment doses consumed to achieve success. This included the cost of rFVIII and rituximab for ITI, PCC and rFVIIa for treatment of breakthrough bleeding, and IVIG for infection prevention in IS patients. The rituximab and IVIG were calculated based on the actual IS-using rate in 2 groups. Additionally, PCC and rFVIIa were calculated based on the actual using-rate in all breakthrough bleedings.
Trial registration number: ChiCTR2200056603. This was the interim analysis. The first participant was enrolled on March 2022.
Results
Total 31 patients (16 in MD-, 15 in LD-ITI group) from 3 hemophilia centers with median followed-up 9 months since ITI-start (Table 1). A total 26 (83.9%) patients underwent peripherally inserted central catheter (PICC) (14 in MD-, 12 in LD-ITI group).
A total 10 (32.3%, 2 in MD-, and 8 in LD-ITI group) patients received IS at median 2.9 months since ITI-start. Among them, 6 patients added IS because of inhibitor titer ≥200BU/mL during ITI, and the rest for delayed-occurred peak-titer or unsatisfactory decrease of titer.
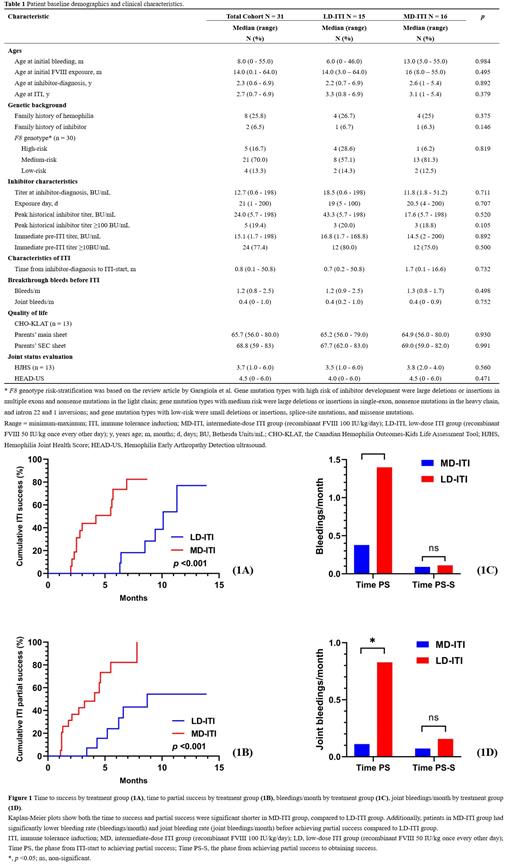
The MD- and LD-ITI group achieved similar success rate (75% vs. 40%). None success patients relapsed over a median follow-up period of 5.1 months since achieving success. The Kaplan-Meier curves demonstrated that patients in MD-ITI group had significantly shorter time to success (median, 2.9 months vs. 9.0 months), and partial success (2.4 months vs. 5.7 months). (Figure 1A and Figure 1B)
Patients in MD-ITI group had lower mean bleeding rate (bleedings/month) (0.38 vs. 1.40) and joint bleeding rate (0.11 vs. 0.83) before achieving partial success, compared with those in LD-ITI group (Figure 1C and Figure 1D). But during the phase from achieving partial success to achieving success, the 2 groups had similar bleeding rate (0.09 vs. 0.11) and joint bleeding rate (0.07 vs. 0.16).
A total 6 (23.1%) patients occurred PICC-related complications (5 in MD-, 1 in LD-ITI group) with mean follow-up period of 270 days. All complications including infection (1 person-time), thrombosis (1 person-time), rashes (3 person-time), accidental detachment (3 person-time).
Similar per kg treatment cost from ITI-start to ITI success was found in 2 groups (US$ 2905.18 vs. US$ 2838.23).
Conclusions
Similar success rate was found in LD-ITI (rFVIII) strategy and MD-ITI (rFVIII) strategy for SHA children with HTI. On the premise of similar cost, shorter time to success, less bleedings were observed in MD-ITI strategy. PICC implantation is a safe option to conduct ITI.
Disclosures
No relevant conflicts of interest to declare.